Research
The following is a summary of some of my most recent research.
Nano Impact Electrochemistry
Suspension of nanoparticles is an intriguing system. The random motion of individual particles caused by Brownian motion may be observed electrochemically by immersing a microelectrode that has been charged to a sufficient potential to transform itself (transformative nano impact) or catalyze electrochemical reactions (catalytic nano impact). With an aid of electrochemical and mass transport models, certain information about physico-chemical properties of nanoparticles and solvation can be obtained.
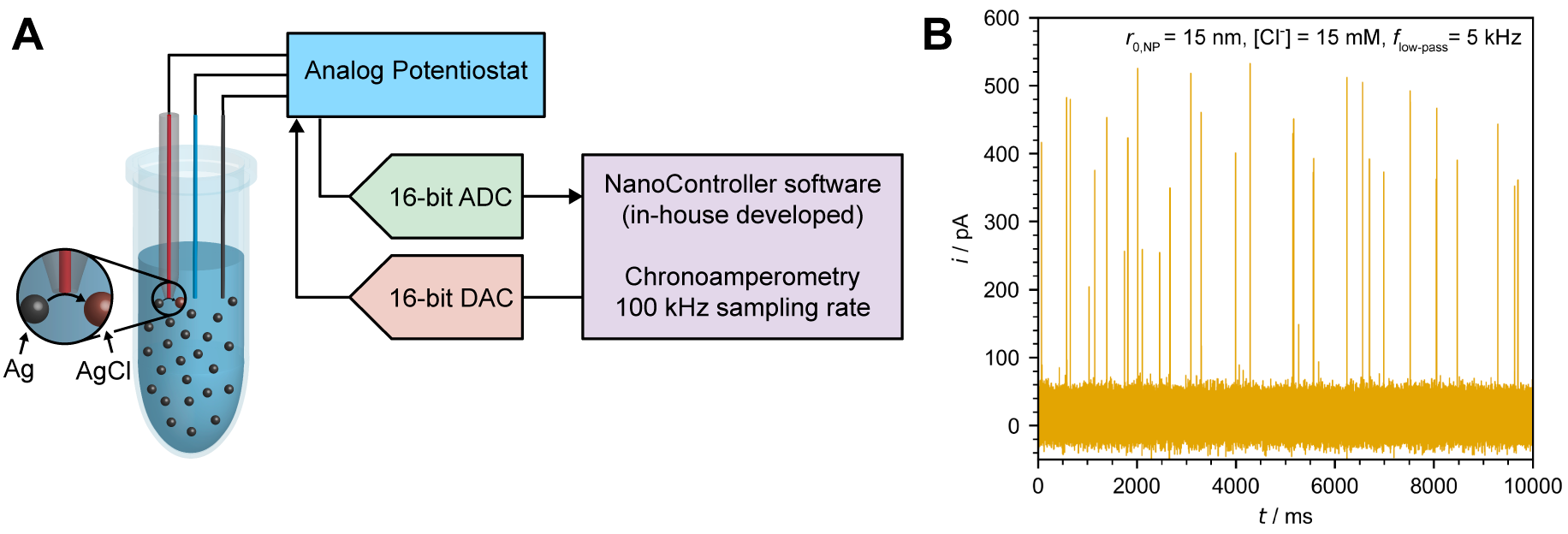
With a sufficient overpotential, the oxidative nano impact of silver nanoparticles in halide solution is controlled by mass transport of halide ions. By precisely measure the time required to oxidize each silver nanoparticle during the impact, we can calculate the diffusion coefficient of halide ions. We demonstrated that this alternative approach is applicable to a wide variety of anions, also with different temperatures and electrolyte compositions.[1]
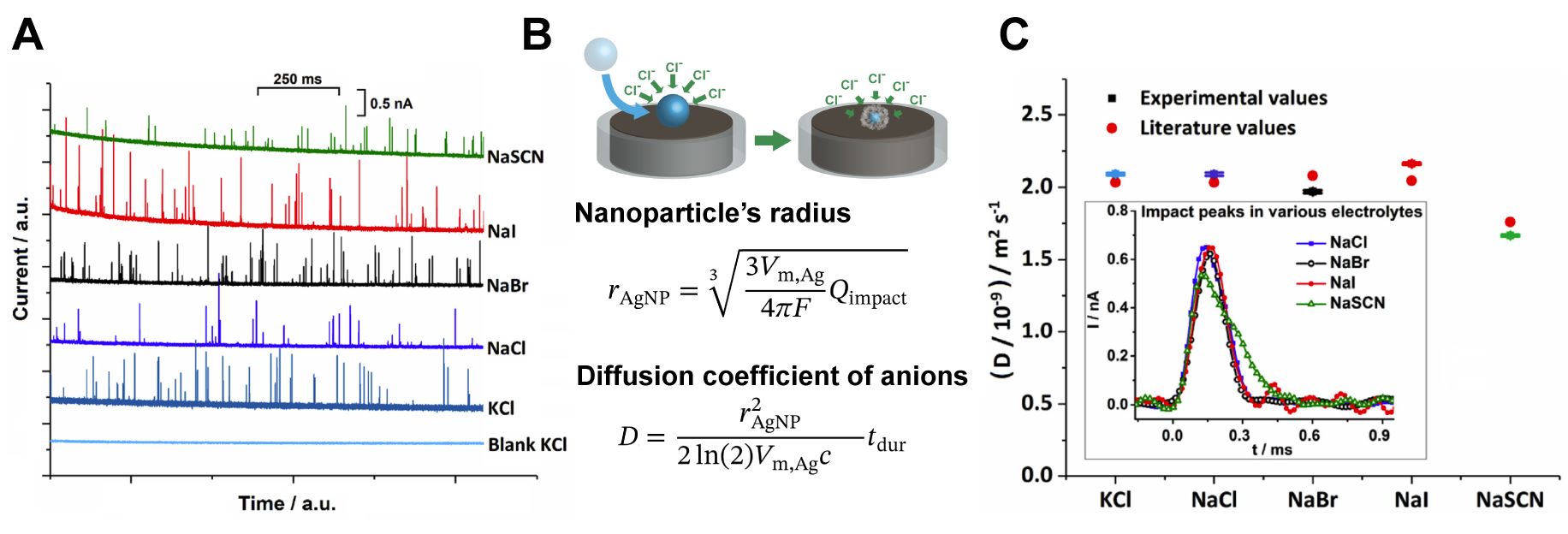
One particularly interesting system is water-alcohol mixtures, in which the solvent molecules differ in size and hence directly alter bulkiness of the solvation shell. The difference in the movement of anions in different solvent compositions could be observed by measuring the change in nanoparticle impact signals.[2]
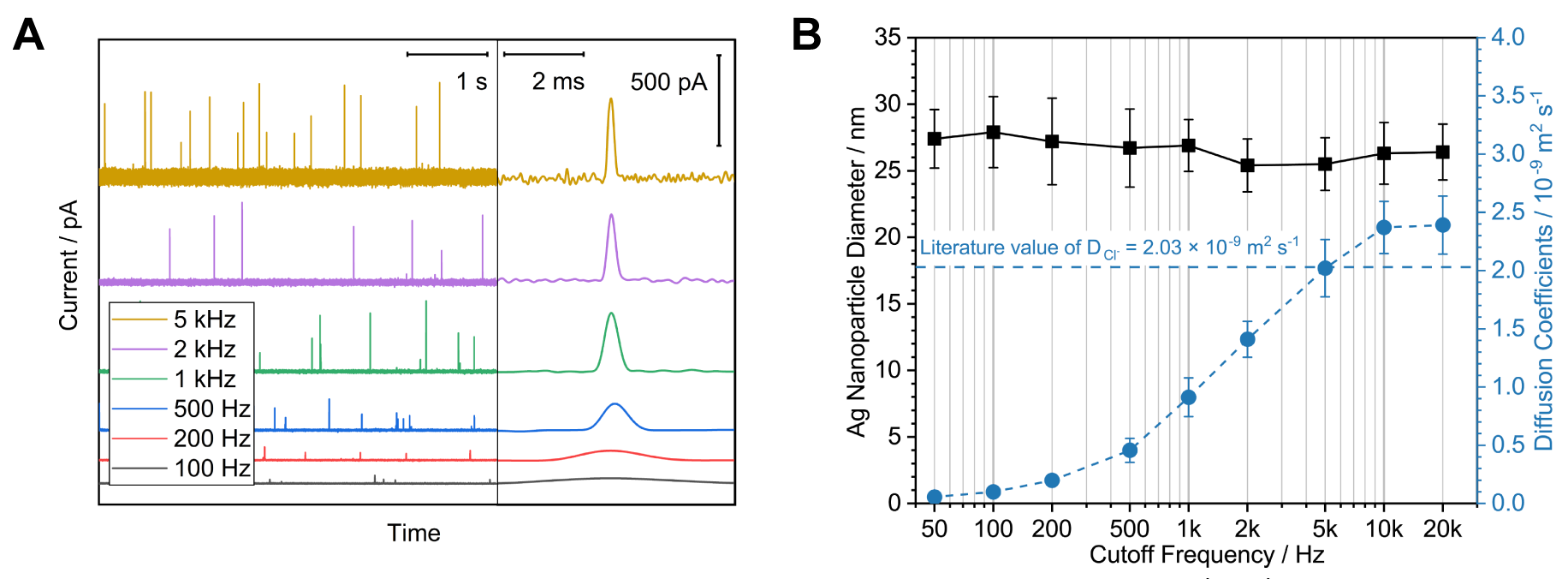
Due to the small current magnitude and pulse-like character of nano impact current, detecting these signal with minimal distortions is difficult and highly dependent of the instrument employed in the experiment. To ensure the signal being monitored is reliable, I developed a methodology for validating the nano impact measuring setup. It was found that while the peak charge is well-conserved even under significant distortions, the measured peak height and duration must be treated with care due to their substantial dependency on the bandwidth of the utilized potentiostats.[3]
To my mind, the only option to conduct reliable and advance nano impact electrochemistry research is to develop in-house electrochemical instruments capable of performing such a unique and challenging measurement. This unplanned objective (which falls beyond the scope of my doctorate proposal) has evolved into one of the major parts of my research.
References
[1] E. N. Saw, N. Blanc, K. Kanokkanchana, K. Tschulik, Electrochimica Acta, 2018, 282, 317-323.
[2] E. N. Saw, K. Kanokkanchana, Hatem M. A. Amin, K. Tschulik, ChemElectroChem, 2022.
[3] K. Kanokkanchana, E. N. Saw, K. Tschulik, ChemElectroChem, 2018, 5, 3000-3005.
Instrumentation Development
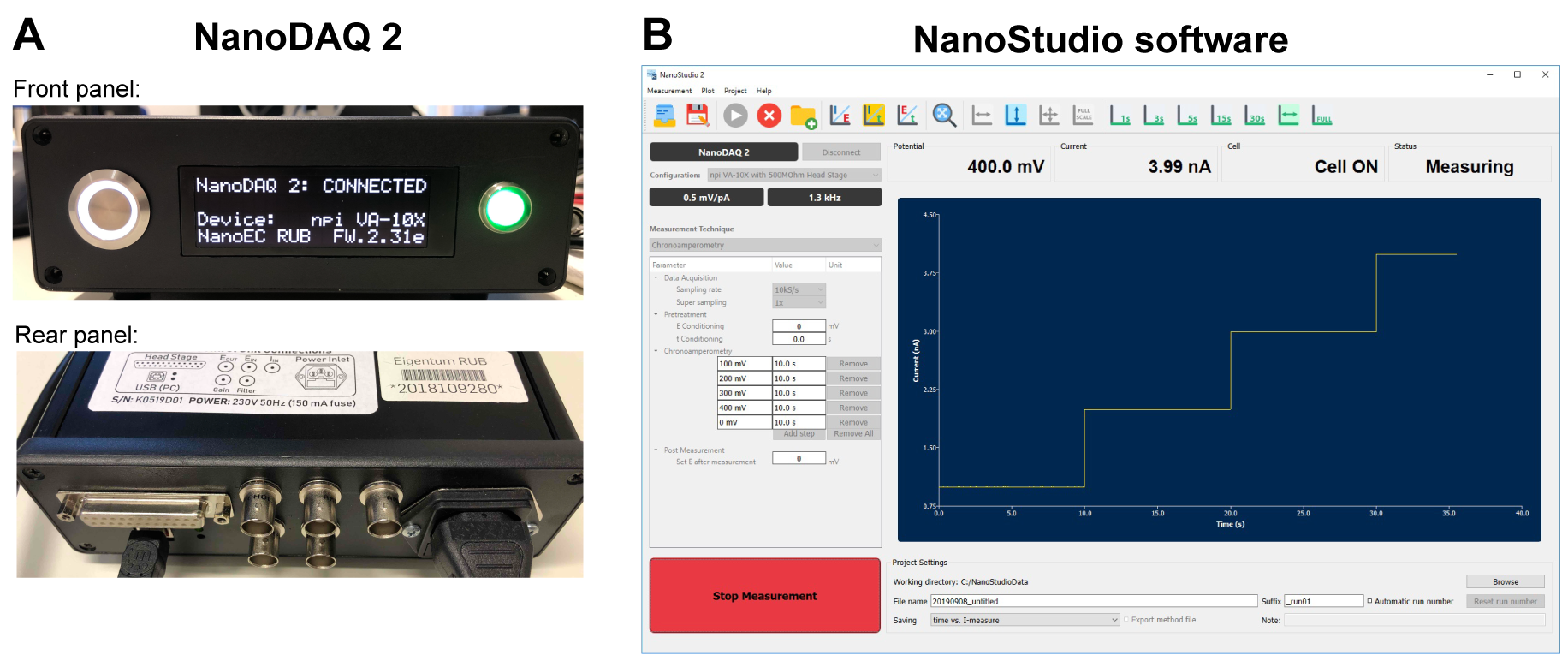
Data acquisition system for high-speed electrochemical measurements
It is remarkable how much information can be extracted from single-entity electrochemistry over such a tiny time scale, e.g., during a single nano impact event. However, this kind of measurement needs an exceptionally fast data acquisition system and electrochemistry software that are not commercially accessible, necessitating the creation of specialized equipment, control firmware, and software in-house. My research involved developing such application-specific and powerful electrochemical workstations.
For nano-impact electrochemistry, where each electrochemical impact spike might be as brief as sub-millisecond, data acquisition rate of up to a few hundred thousands data point per second is necessary. This requires specialized hardware and software that function in unison. To achieve this, I created the NanoDAQ2 and the accompanying NanoStudio software. Both are designed for simultaneous electrochemical signal sampling at a rate of up to 200 ksps. With an 18-bit resolution, expandability for customized designed low-current potentiostat modules, and data visualizations enhanced by OpenGL. Nano impact measurements can be made relatively simple with a high degree of reliability.
Data acquisition at an extreme rate (up to a few hundred thousands data point per second) with real-time data visualization is required for nano-impact electrochemistry in which each electrochemical impact spike can be as short as sub-millisecond. This requires special hardware and software which synchronously work together. For this purpose, I developed the NanoDAQ2 and the complimentary NanoStudio software. Both of which are optimized for simultaneous sampling of electrochemical signals at a rate up to 200 ksps. With 18-bit resolution and OpenGL accelerated data visualizations. Nano-impact measurements can be made relatively simple and reliable.
Electrochemical platform for material research and electrochemical sensor development
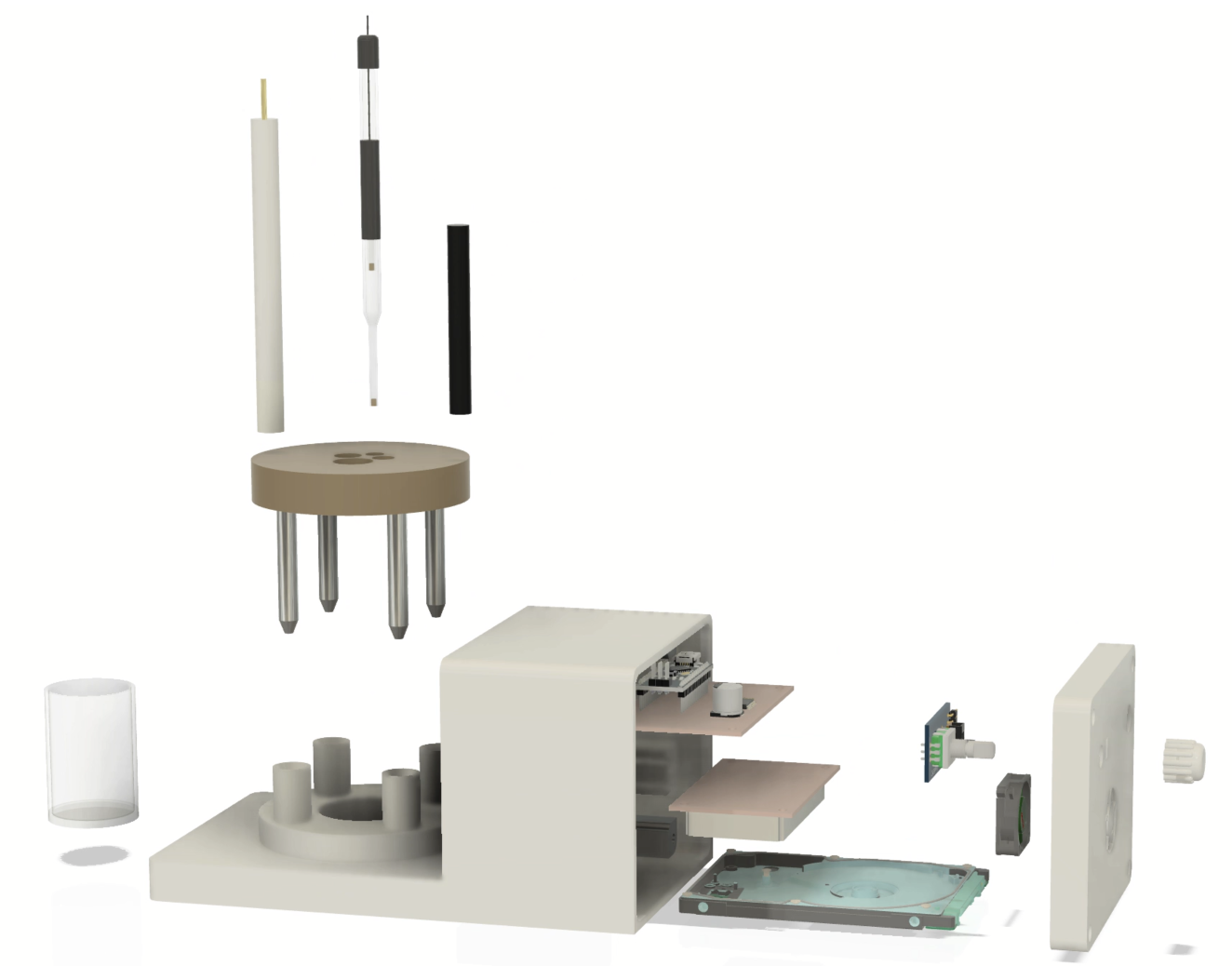
Depending on target applications, the ability to design and manufacture well-defined electrochemical cell and setups with great control benefits research significantly. I have developed several prototypes for my research; the one seen below is a dedicated setup for nanoparticle electrocatalysts' ensemble studies. Due to the system's well-defined dimensions and precise convection control capabilities, electrochemical experiments can be conducted with a high degree of repeatability. Thanks to the advent of 3D printing technology, this setup is easy to create and requires only some basic CAD design skills.